Services Available
Intracytoplasmic sperm injection (ICSI)
Intracytoplasmic sperm injection (ICSI) is an in vitro fertilization procedure recommended for mares that have not been able to establish a pregnancy with conventional insemination techniques, or when there is interest in obtaining embryos in periods when they are competing. The technique can also be very efficient in generating embryos from limited sperm samples with high economic value, offering higher success rates than those that can be obtained with traditional insemination methods. The ICSI technique is offered commercially in partnership with veterinarians across Europe with proven experience in OPU. Once the oocytes have been extracted from the mare, they are sent to our laboratory, where they are matured in vitro and subsequently inseminated. The blastocysts resultant from the ICSI sessions can then be vitrified and later on shipped to any reproductive center to be transferred. We are flexible in scheduling the ICSI sessions, however, it is preferable to receive oocytes during the weekdays to avoid shipping and courier problems that sometimes occur on weekends. We collaborate with reference centers (e.g., http://brancaleoneteam.com) that have a large catalog of samples from the best stallions in the world with the possibility of being used in ICSI sessions.
Personalized training sessions in OPU can be arranged to veterinarians who intend to implement the OPU technique in their services portfolio. The training is offered by internationally renowned veterinarians who have been pioneers in the development and application of the technique in the most prestigious equine breeding centers worldwide.
Embryo biopsy for genetic diagnosis
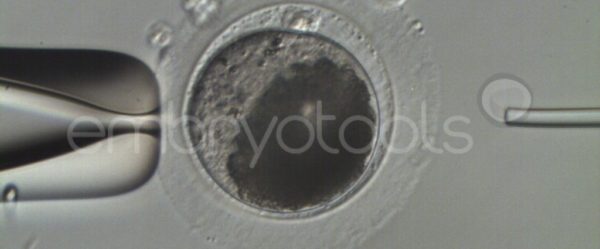
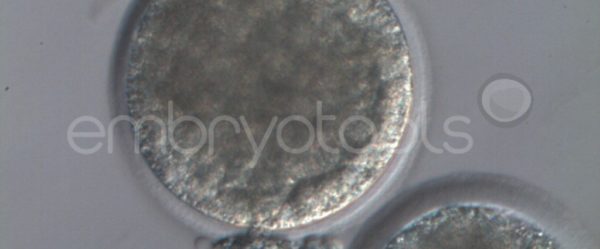
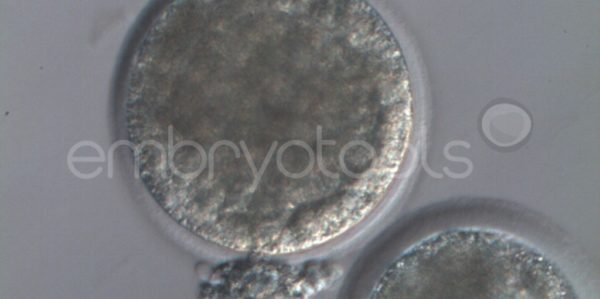
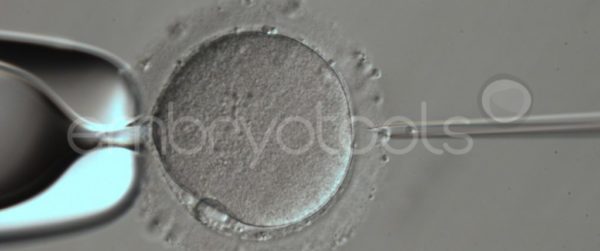
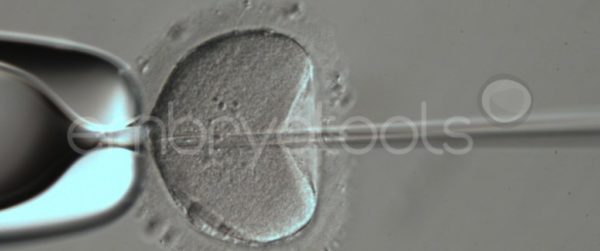
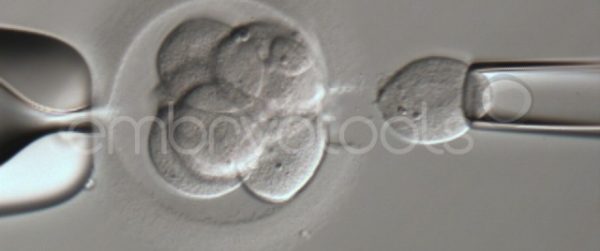
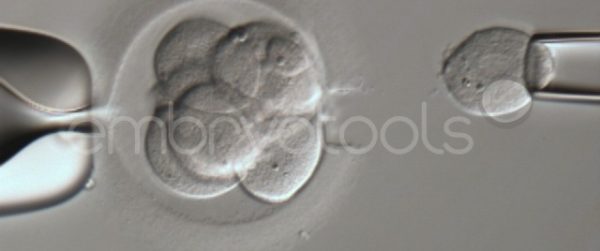
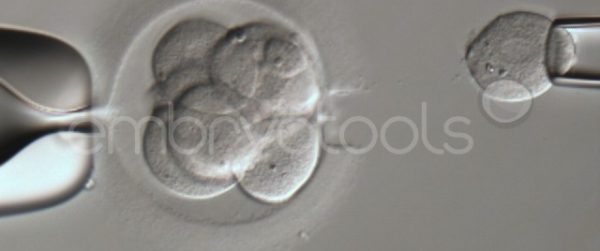
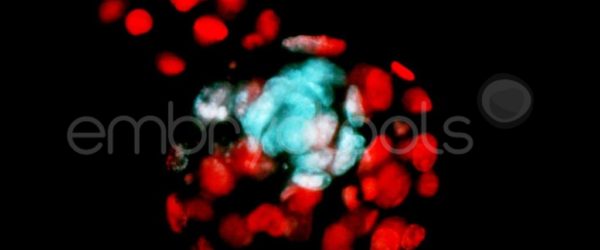
Embryo biopsy is based on the extraction of a reduced number of cells from an embryo at an early stage of development, without compromising its later development. The biopsied cells are immediately processed for genetic analysis and the results are obtained before the embryo is transferred to a recipient. Using this technique, it is possible to select the sex or color of the future animal as well as to avoid the transfer of an embryo carrying a mutation or chromosomal abnormality that causes a disease. The technique can be applied to blastocysts obtained from insemination or by ICSI.
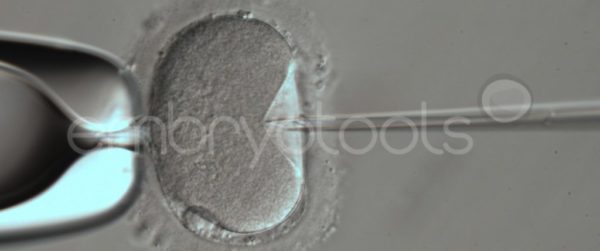
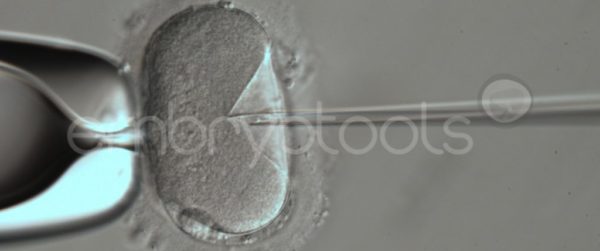
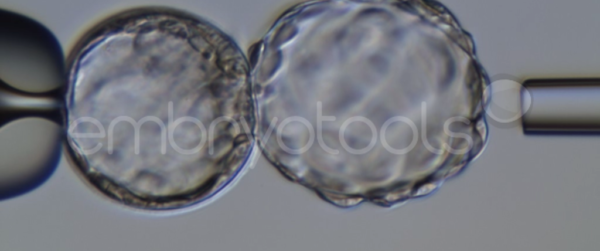
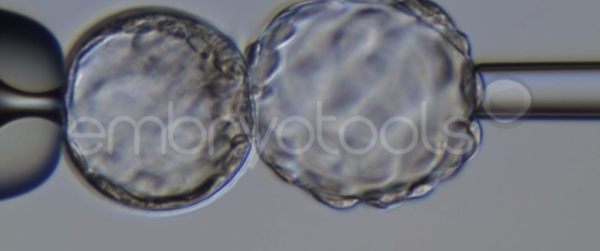
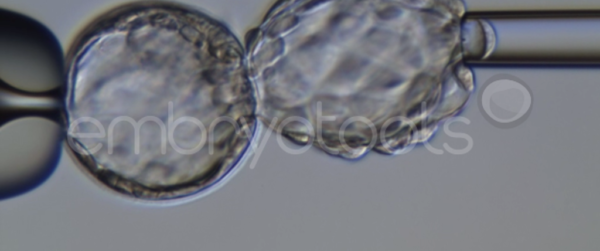
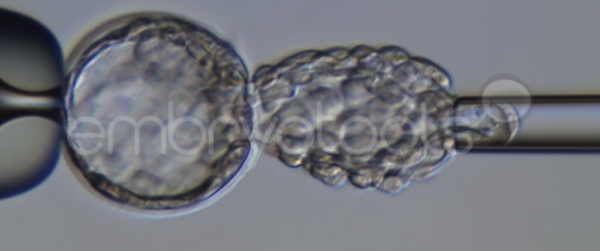
Blastocyst collapse and vitrification
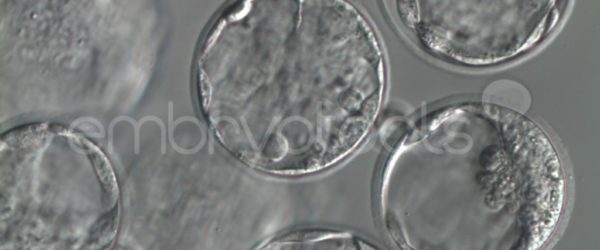
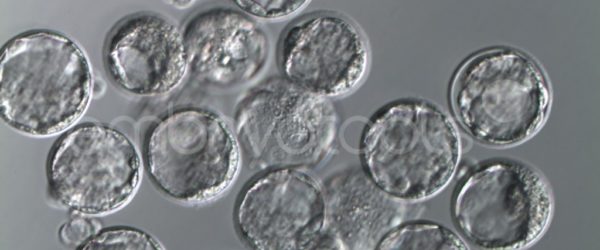
We have extensive experience in the most advanced embryo vitrification methods. Embryos can be cryopreserved at different stages of development with high success rates and stored in liquid nitrogen indefinitely until our clients decide to transfer them to recipients. In the case of large size blastocysts (> 300-1500 µm), a micromanipulation technique is applied that allows the collapse of the embryo prior to vitrification, increasing success rates.
Genetic preservation