The results from the first clinical pilot study of maternal spindle transfer (MST) were published online in the journal, Fertility and Sterility, as an ahead of print version of the article. The project was carried out in Greece, at the Institute of Life-IASO IVF Center, and involved a multidisciplinary team of scientists from internationally renowned institutions: Embryotools (Science Park, Barcelona, Spain); Juno Genetics (UK); the University of Oxford (UK); Oregon Health & Science University (US). The exploratory study provides the first insights into safety and efficacy of maternal spindle transfer in humans, when applied in a context of infertility treatment. The study resulted in the birth of six children to patients with a long history of previous failed in vitro fertilization attempts. The article additionally reveals important information concerning the potential use of MST to reduce the risk of disease transmission in patients carrying pathogenic mitochondrial DNA (mtDNA) mutations.
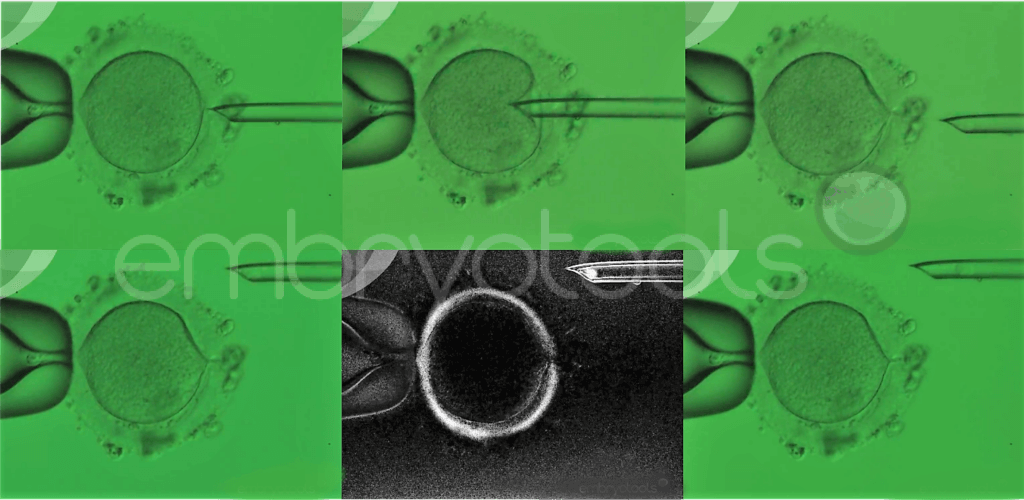
The egg is the most important element during the first few days of life. Not only does it carry the genetic contribution (DNA) from the mother, but it also contains stores of materials (e.g., RNA, protein, energy supplies and organelles) in its cytoplasm vital for the developing embryo. Poor egg quality is a significant factor contributing to female infertility, for which no effective treatments have been developed. The problem is characterized by repeated failure of eggs to fertilize and/or impaired embryo development. Currently, the only strategy available for patients that produce poor quality eggs is to undergo in vitro fertilization (IVF) treatments using donated eggs or embryos. This approach can help patients achieve a pregnancy, but excludes them from a genetic contribution to their child.
Maternal spindle transfer is an advanced laboratory technique that belongs to the family of methods known collectively as mitochondrial replacement therapies (MRTs). These techniques were originally proposed to avoid the transmission of mitochondrial diseases and their application for this clinical purpose is already permitted in some countries, such as the UK and Australia. The method involves the replacement of the patient’s egg cytoplasm with cytoplasm taken from young donated egg, while retaining the patient’s nuclear genetic material. Accumulating evidence suggests that this process can overcome some problems related to a failure of an egg to support fertilization and embryonic development, while also allowing patients to produce genetically related offspring.
This exploratory pilot study was conducted in Greece after receiving approval from the National Authority of Assisted Reproduction. The research team aimed to explore, for the first time, the clinical feasibility of the maternal spindle transfer technique in a context of infertility treatment. The pilot study started in 2018 and was limited to a cohort of 25 infertile couples that were carefully selected based on their long history of unsuccessful IVF treatments, associated with poor egg quality. The patients had undergone between 3 and 11 previous IVF attempts (average 6.4 per patient) without success. The outcomes monitored in the study included the usual measures of IVF success, as well as other parameters specifically related to the technique, and pediatric follow-up to evaluate the general health of children born following the procedure.
The data obtained during the study is unique, suggesting that the maternal spindle transfer technique might have the potential to help a class of infertile patients that has been extremely difficult to treat with conventional methods. Together, the patients included in the study had undergone a total of 159 previous IVF treatments, in which 423 mature eggs had been collected, but no pregnancies had ever been achieved. A total of 28 maternal spindle transfer attempts were carried out, resulting in the birth of six babies. The health and developmental status of the children (some now close to 4 years old) is unremarkable, providing some reassurance about the safety of the method.
The scientific team monitored the amount of DNA from mitochondria (mtDNA) transferred into the donor egg along with the patient’s spindle and showed that more than 99% of the mtDNA in the embryos produced was from the egg donor. However, in one child born following the procedure the mitochondria from the patient expanded dramatically during development, and by the time of birth had come to represent about 50% of the total in the cells of the child. This is the first time this phenomenon, known as ‘reversal’ has been reported in human embryos. While none of the patients in the study were carriers of mitochondrial disease, the possibility that the small number of mitochondria, unavoidably transferred to the donor oocyte along with the patient’s DNA, could proliferate disproportionately has implications for the use of MRTs to prevent the transmission such disorders. The severity of mtDNA disorders is linked to the proportion of mitochondria derived from the affected patient. The resurgence of a patient’s mitochondria, after they were initially reduced to a tiny population, suggests that some of these treatments might be less than 100% effective.
While the data obtained is encouraging, potentially creating a new therapy for types of infertility that were previously untreatable, the researchers are keen to stress that this was a pilot study, and as such was limited in size and scope. A definitive assessment of the clinical value of the technique must await future larger, controlled and randomized trials.
___________________________
Article identification: DOI: https://doi.org/10.1016/j.fertnstert.2023.02.008.
Authors: Nuno Costa-Borges, PhD; Eros Nikitos, MSc; Katharina Späth, PhD; Irene Miguel-Escalada, PhD; Hong Ma, PhD; Klaus Rink, PhD; Clement Coudereau, PhD; Hayley Darby; Amy Koski, MSc; Crystal Van Dyken, PhD; Enric Mestres, PhD; Evmorfia Papakyriakou, MSc; Dominique De Ziegler, MD; George Kontopoulos, MD; Themistoklis Mantzavinos, MD; Ioannis Vasilopoulos, MD; Stylianos Grigorakis, MD; Thomas Prokopakis, MD; Konstantinos Dimitropoulos, MD; Panagiotis Polyzos, MD; Nikolaοs Vlachos, MD; Konstantinos Kostaras, MD; Shoukhrat Mitalipov, PhD; Gloria Calderón, PhD; Panagiotis Psathas, MD; Dagan Wells*, PhD.
Contacts:
Dr. Nuno Costa-Borges
Embryotools, Barcelona, Spain
nuno.borges@embryotools.com
Prof. Dagan Wells
Juno Genetics / University of Oxford
dagan.wells@junogenetics.com
Dr. Shoukhart Mitalipov
Oregon Health & Science University
mitalipo@ohsu.edu
Dr. Panagiotis Psathas
Institute of Life-IASO IVF Center
psath438@otenet.gr

